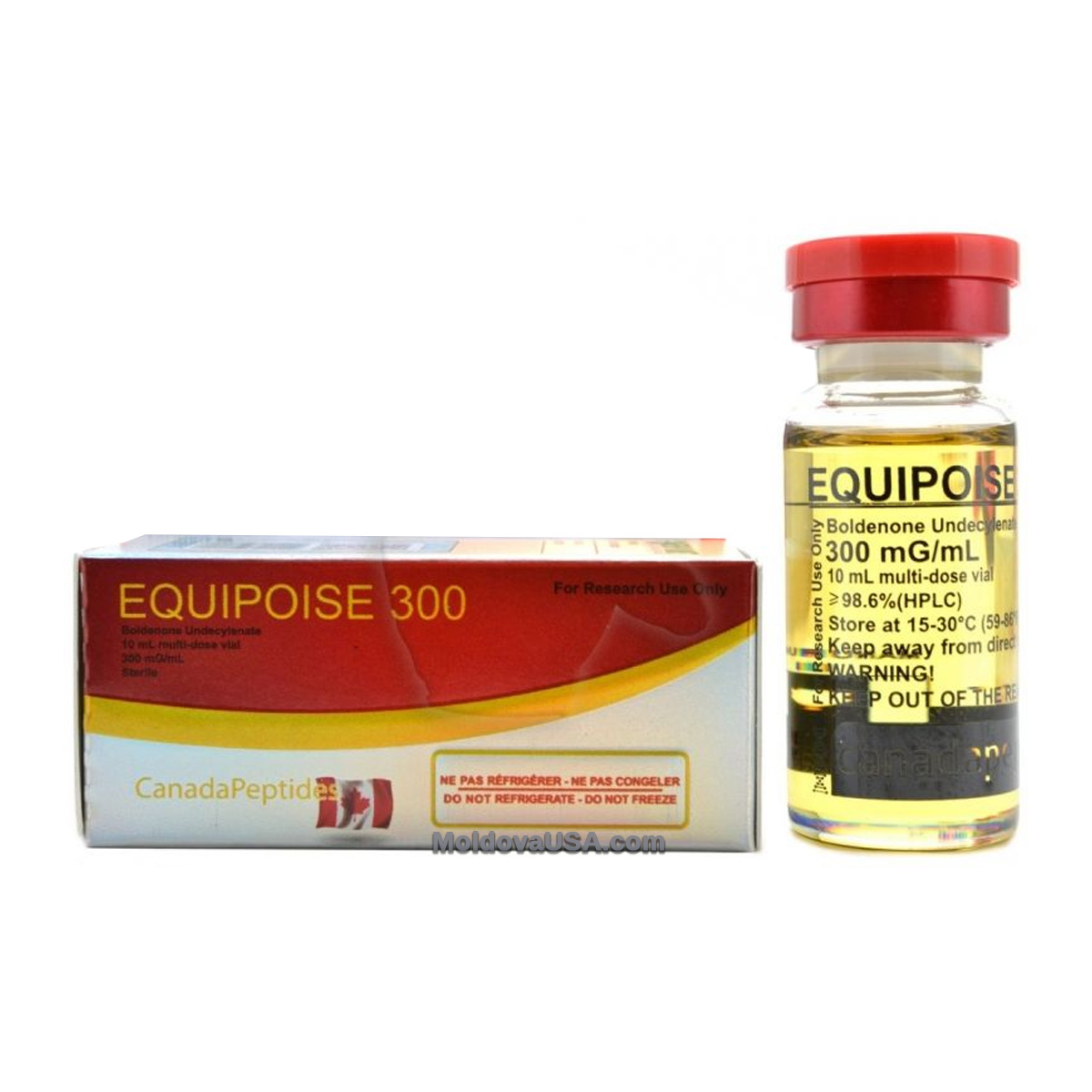
Description
History of Equipoise
Boldenone is an oil-based injectable anabolic steroid with the Undecylenate ester linked for a longer release rate and half-life. It is an anabolic derivative of Testosterone with a decreased androgenic impact. Equipoise is a veterinary product designed for use on animals. Equipoise is a less androgenic version of Testosterone with less Estrogenic action than Testosterone. Many users like to compare it to Nandrolone (Deca Durabolin), however this is a bad comparison since Nandrolone has different characteristics than Equipoise and comes from a different family of chemicals (a 19-nor, also known as a progestin). Many believe Nandrolone and Equipoise to be interchangeable in a cycle.
An interesting tidbit about this compound: Equipoise was created in 1949, long before Dianabol (Methandrostenolone), making it the first synthetic derivative of Testosterone. Furthermore, Dianabol is Equipoise (Boldenone) with a methyl group added to its 17-beta hydroxyl group (also known as C17-alkylation) to make it orally accessible in the body. Dianabol (Methandrostenolone) is really Equipoise C17-alkylated. The methylation at the 17th carbon atom altered enough characteristics of the molecule that it was deemed a distinct anabolic steroid analogue, and called Methandrostenolone (Dianabol). The methyl group attached to the 17th carbon on the Dianabol chemical structure is the only difference between the two. It is clear that these two anabolic steroids are almost identical.
Although Equipoise was created in 1949, it wasn’t commercialized until the 1960s as Parenabol. Between 1949 until its introduction in the 1960s, Ciba experimented with Equipoise, trying to improve its half-life and release rates. The Undecylenate ester was added and the final preparation was Boldenone Undecylenate. This chemical was tested clinically in the late 1960s and early 1970s as an anabolic steroid that would promote lean mass and prevent weight loss in patients with wasting and osteoporosis. Parenabol was removed off the market in the late 1970s due to lack of applicability and usage.
Squibb would subsequently acquire the patents for Boldenone and re-release it as Equipoise, a veterinary anabolic steroid designed for use in horses but also in other animals. Equipoise is renowned for its lean mass gains and appetite-stimulating effects, which are seen in virtually all anabolic steroids. However, Equipoise tends to increase hunger more than other anabolic steroids. Although the manufacturing and marketing of Equipoise has changed several times over the years, it is still accessible on the American and worldwide markets under various generic and brand names.
Equipoise chemistry
As previously stated, Equipoise is a synthetic derivative of Testosterone that has double-bonds inserted between carbon 1 and carbon 2 on the steroid molecule. This is the alteration that reduces Equipoise’s propensity for interacting with the aromatase enzyme (the enzyme responsible for the conversion of androgens into Estrogen). As a consequence, it has less Estrogenic action than Testosterone. Boldenone with an Undecylenate ester added. Undecylenate is Undecylenoic acid, however when linked to Boldenone it is an ester bond (or ester linkage). Boldenone’s 17-beta hydroxyl group is linked to undecylenoic acid. A prolonged release window is achieved by increasing the hormone’s release rate and half-life. Enzymes work to dissolve the ester-hormone link after Boldenone Undecylenate reaches the circulation, which takes time. These enzymes remove the ester from the hormone, resulting in pure Boldenone that can function in the body. The enzymes removing the ester from the hormone is what causes the slower release rates. Boldenone Undecylenate is created by attaching the Undecylenate ester to Boldenone, extending its half-life to 14 days.
The only structural difference between Equipoise and Dianabol is that Equipoise has an Undecylenate ester connected to its 17-beta hydroxyl group whereas Dianabol has a methyl group attached to it. The methyl group (also known as C17-alkylation) prevents the liver from metabolizing and breaking down Dianabol when taken orally. Both hormones are identical except for these minor changes. However, both of these hormones function substantially differently in the body, indicating that adding a methyl group (C17-alkylation) to the 17th carbon affects more than simply the hormone’s resistance to breakdown in the liver.
Equipoise properties
Equipoise has several characteristics with Testosterone as a derivative. As an aromatizable anabolic steroid, Equipoise may and does transform into Estrogen in the body when it interacts with the aromatase enzyme. In the body, its double-bond alterations between carbons 1 and 2 decrease its affinity for the aromatase enzyme.
Equipoise will still convert to Estrogen, although in far less amounts than its parent hormone Testosterone. However, the problem of Estrogenic side effects remains and should not be disregarded by any consumers. Thus, Equipoise alone will not eliminate possible water retention, but will greatly reduce it. While Equipoise should not cause bloating or other estrogenic side effects at reasonable and moderate dosages (depending on user sensitivity), the risk of these side effects increases with increasing Equipoise doses. Increasing dosages will enhance the rate of aromatization into Estrogen.
For those who are sensitive to androgenic side effects, Equipoise has a lower androgenic strength rating than its progenitor hormone Testosterone. Overall, Equipoise provides the similar anabolic gains in size, strength, and muscle as Testosterone, but with less estrogenic and androgenic adverse effects. Equipoise should provide excellent lean mass gains with little water retention (depending on dosage) and be a great complement to any bulking or lean mass cycle. It may potentially replace Nandrolone in a stack.
Doping use
Boldenone is mostly anabolic, with minimal androgenic potential. This is due to the undecylenate ester linked to the parent steroid.
Boldenone promotes nitrogen retention, protein synthesis, hunger, and erythropoietin release in the kidneys. The medication is widely used in bodybuilding doping, which is prohibited. To help in bodybuilding, the medication is taken in a steroid stack with other anabolic steroids, typically with testosterone as the ‘base’.
Bodybuilders utilize Boldenone both off-season and pre-contest. It is used to increase strength and size since it is comparable to methandienone (methylated boldenone). Boldenone also stimulates hunger, which is why many athletes avoid it before to competition. Bodybuilders use Boldenone to increase their vascularity.
In the sport of bodybuilding, common dosages vary from 200 mg to 400 mg/week.
Boldenone has a modest aromatization rate (about 50% of testosterone), thus it does not readily convert to estrogen and does not induce water retention. It is an excellent Nandrolone substitute for many bodybuilders.
Boldenone is among the drugs prohibited by MLB and most other major sports leagues.
Seen in blood tests owing to extended metabolic half-life. Months after stopping usage, traces of the medication may be found.
Side effects
Unlike nandrolone, boldenone has no progesterone receptor interaction and no progesterone-like adverse effects. Boldenone has moderate androgenic side effects. This chemical may cause oily skin, acne, increased aggressiveness, and hair loss. Boldenone is converted to the more powerful androgen dihydroboldenone by the 5-alpha-reductase enzyme, although its affinity for this interaction in the human body is minimal to non-existent. Boldenone’s adverse effects include suppression of the HPTA, water retention, acne flare-ups, potential estrogen conversion, and high blood pressure. Boldenone is known to induce anxiety and flu-like symptoms in short ester forms like acetate and propionate (nearly useless), but also in enanthate, cypionate, and undecyclenate.
DOSAGE PACKAGING
300mg/ml 10ml vial /box
Description
Boldenone undecylenate, or boldenone undecenoate, is an androgen and anabolic steroid (AAS) medication which is used in veterinary medicine, mainly in horses.It was formerly used in humans as well.
Chemical name
[(8R,9S,10R,13S,14S,17S)- 10,13-Dimethyl-3-oxo-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta [a]phenanthren-17-yl] undec-10-enoate
Synonyms
Boldenone undecylenate
13103-34-9
Parenabol
17b-[(1-Oxo-10-undecenyl)oxy]-androsta-1,4-dien-3-one
Vebonol
MeSH Entry Terms
17 beta-hydroxyandrosta-1,4-dien-3-one 10-undecenoate
3-oxo-1,4-androstadiene-17-ol undecylenate
Ba 29038
boldenone undecylenate
Equipoise
Parenabol
Chemical and physical data
Formula
C30H44O3
Molecular Weight
452.679 g·mol−1
Appearance
Solid powder
Melting point
32-39 °C
Boiling point
553.2±50.0 °C at 760 mmHg
Purity
>98%
Density
1.1±0.1 g/cm3
Vapour Pressure
0.0±1.5 mmHg at 25°C
Enthalpy of Vaporization
83.4±3.0 kJ/mol
Index of Refraction
1.537
Molar Refractivity
134.1±0.4 cm3
Flash Point
232.2±30.2 °C
Solubility
Soluble in DMSO, not in water
Polar Surface Area
43 Å2
Polarizability
53.1±0.5 10-24cm3
Surface Tension
41.7±5.0 dyne/cm
Molar Volume
428.9±5.0 cm3
Hazard Codes
Xn
Identifiers
CAS Number
13103-34-9
IUPAC name
[(8R,9S,10R,13S,14S,17S )-10,13-Dimethyl-3-oxo- 6,7,8,9,11,12,14,15,16,17- decahydrocyclopenta[a] phenanthren-17-yl] undec- 10-enoate
UNII
ZS6D2ITA30
PubChem CID
11954310
IUPHAR/BPS
DrugBank
DBSALT001616
ChemSpider ID
10128605
KEGG
D03145
CHEBI
ChEMBL
2106059
InChI
1S/C30H44O3/c1-4-5-6-7-8 -9-10-11-12-28(32)33-27-16-15-25 -24-14-13-22-21-23(31)17-19- 29(22,2)26(24)18-20- 30(25,27)3/h4,17,19,21, 24-27H,1,5-16,18,20H2,2- 3H3/t24-,25-,26-,27-,29 -,30-/m0/s1
InChIKey
AHMMSNQYOPMLSX-CNQKSJKFSA-N
DSSTox Substance ID
DTXSID00156854
CompTox Dashboard
DTXSID00156854
ECHA InfoCard
100.032.734
Smiles
C[C@]12CC[C@H]3[C@H] ([C@@H]1CC[C@@H]2O C(=O)CCCCCCCCC=C)CC C4=CC(=O)C=C[C@]34C
Canonical SMILES
CC12CCC3C(C1CCC2OC( =O)CCCCCCC CC=C)CCC4 =CC (=O)C=CC34C
Isomeric SMILES
C[C@]12CC[C@H]3[C@H] ([C@@H]1CC[C@@H]2OC (=O)CCCCCCCCC=C)CC C4=CC(=O)C=C[C@]34C