Description
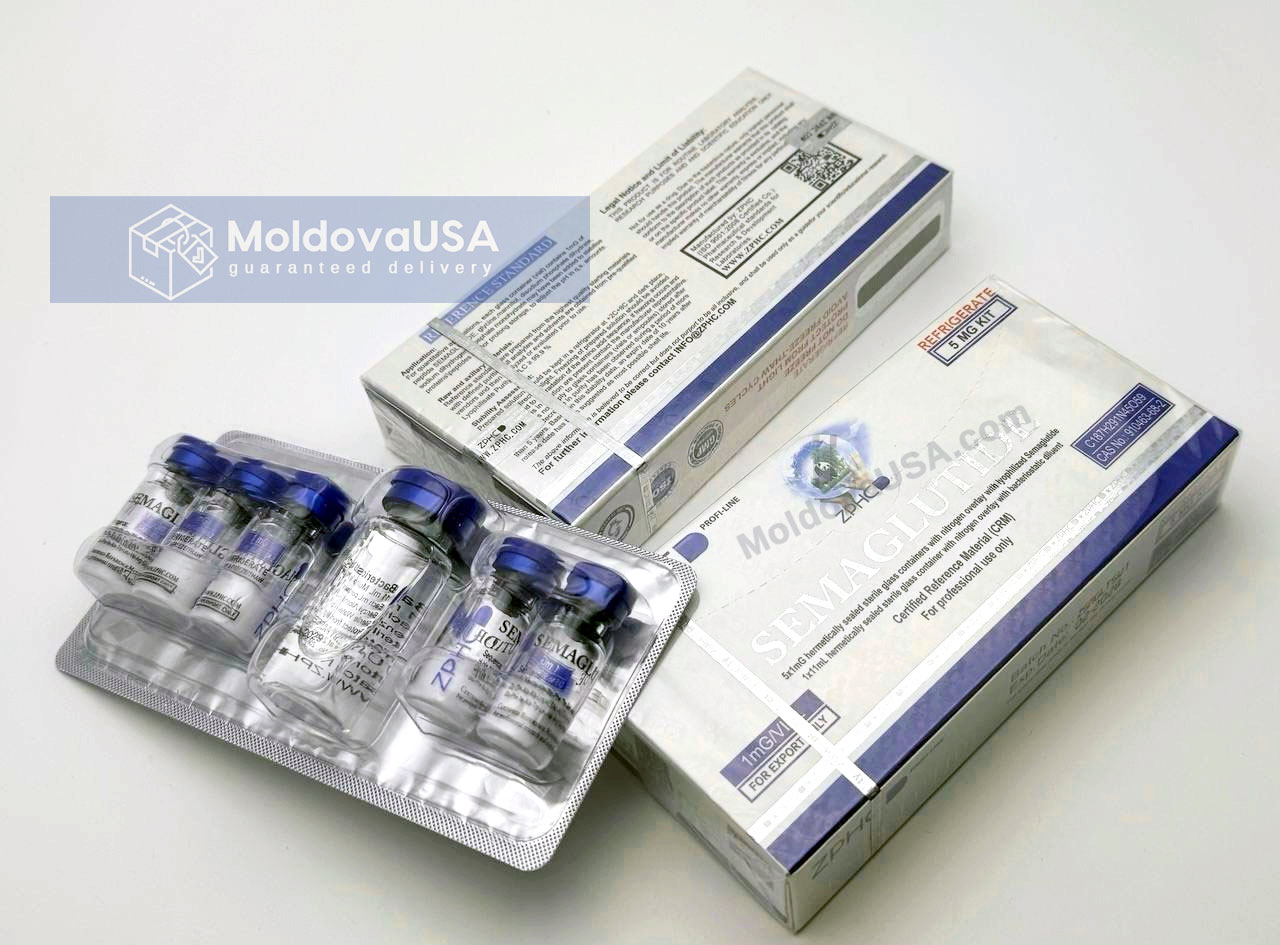
Discover new product that works like Ozempic, now available in our store MoldovaUSA. The Zhengzhou Semaglutide 1mg 5 Vial Kit offers an effective solution for managing blood sugar levels. Order now and take advantage of the latest in medical treatments.
Pharmacological Action and Usage Guidelines
Semaglutide is an agonist of the GLP-1 receptor (GLP-1R), produced via recombinant DNA biotechnology using a strain of Saccharomyces cerevisiae followed by purification. It is a GLP-1 analog with 94% homology to human GLP-1. Semaglutide acts as a GLP-1R agonist, selectively binding to and activating GLP-1R, which is the target for native GLP-1.
GLP-1 is a physiological hormone with multiple effects on glucose regulation, appetite, and the cardiovascular system. The influence on glucose concentration and appetite is specifically mediated by GLP-1R located in the pancreas and brain. Pharmacological concentrations of semaglutide reduce blood glucose levels and body weight through a combination of the effects described below. GLP-1R is also present in specific areas of the heart, blood vessels, immune system, and kidneys, where its activation may produce cardiovascular and microcirculatory effects.
Indications for Use
ZPHC Semaglutide 1mg 5 vials is indicated for use in adult patients with type 2 diabetes mellitus as an adjunct to diet and exercise to improve glycemic control in the following scenarios:
- Monotherapy.
- Combination therapy with other oral hypoglycemic agents such as metformin, metformin with a sulfonylurea, metformin and/or a thiazolidinedione, in patients who have not achieved adequate glycemic control with previous therapy.
- Combination therapy with insulin in patients who have not achieved adequate glycemic control with Ozempic® and metformin.
ZPHC Semaglutide is also indicated to reduce the risk of major cardiovascular events in patients with type 2 diabetes and high cardiovascular risk as an adjunct to standard treatment for cardiovascular diseases.
Contraindications
- Hypersensitivity to semaglutide or any of the excipients.
- Personal or family history of medullary thyroid carcinoma.
- Multiple endocrine neoplasia syndrome type 2.
- Type 1 diabetes mellitus.
- Diabetic ketoacidosis.
ZPHC Semaglutide is contraindicated in the following patient groups and conditions due to the lack of efficacy and safety data or limited experience:
- Pregnancy.
- Breastfeeding period.
- Patients under 18 years old.
- Severe hepatic insufficiency.
- End-stage renal disease (creatinine clearance <15 ml/min).
- Chronic heart failure (NYHA class IV).
Use with Caution
ZPHC Semaglutide should be used with caution in patients with renal insufficiency and those with a history of pancreatitis.
Dosage and Administration
The initial dose of ZPHC Semaglutide is 0.25 mg once a week. After 4 weeks, the dose should be increased to 0.5 mg once a week. To further improve glycemic control after at least 4 weeks of treatment at 0.5 mg per week, the dose can be increased to 1 mg once a week.
A dose of 0.25 mg is not therapeutic. Doses above 1 mg per week are not recommended.
ZPHC Semaglutide can be used as monotherapy or in combination with one or more hypoglycemic agents. When added to pre-existing metformin and/or thiazolidinedione therapy, these treatments can be continued at their previous doses.
When adding ZPHC Semaglutide to therapy with sulfonylureas or insulin, the dose of sulfonylureas or insulin should be reduced to minimize the risk of hypoglycemia.
Use of ZPHC Semaglutide does not require self-monitoring of blood glucose levels. Self-monitoring is necessary for adjusting the dose of sulfonylureas and insulin, especially at the beginning of treatment and when reducing the dose of insulin. A stepwise approach to insulin dose reduction is recommended.